Advertisement
Advertisement
July 2018
CT Venography: Technique and Indications
A brief summary of how to perform indirect and direct CT venography and when this imaging modality can be helpful in diagnosing venous disease.
CT venography (CTV) is not a particularly well-validated technique, with most data derived from anecdotal experience. For a variety of reasons, CTV has become the go-to imaging modality for a quick and efficient answer to most venous problems. For example, we work in a trauma center with a large oncology population, and we have learned that oftentimes ultrasound simply doesn’t cut it, particularly in the context of intra-abdominal malignancy. Even in very experienced hands, things can be missed. CTV/contrast-enhanced CT provides multiple extra levels of information, including the presence or absence of inferior vena cava (IVC) filters; the condition of the IVC, renal veins, collaterals, and internal iliac veins; potential iliac vein compression or nutcracker syndromes; presence of pelvic varicosities; as well as ovarian vein thrombosis and undiagnosed malignancy.
TECHNIQUE
Indirect CTV
Often combined with CT pulmonary angiography (CTPA) in the acute setting, indirect CTV is performed as a standard, nonoral, post–intravenous contrast-enhanced CT at approximately 120 to 150 seconds after injection of contrast, which is considerably later than portal venous phase. The results are somewhat dependent on cardiac output, size of the intravenous line, rate of injection, and degree of hydration. Indirect CTV images do not look like CTA images, but they are almost always diagnostic (just not quite a “sexy” as those CTAs obtained for endovascular aneurysm repair). For instance, maximum intensity projection techniques are not helpful, as the degree of contrast opacification is inadequate. All the fancy stuff is unnecessary; all you need is a good-quality axial images to opacify the vessel.
Direct CTV
Direct CTV was first demonstrated to our team by Dr. Frédéric Thony and colleagues from Grenoble, France. First, a thigh-high compression stocking is placed on the affected limb, and a 21-gauge needle is inserted into any vein in the foot. Then, 100 mL of iodinated contrast is injected at 3 mL/second with a 30-mL saline chaser, and scans are acquired from mid-calf up to the diaphragm. This technique provides superb detail and is, in our view, essential for planning endovascular reconstruction, especially in postthrombotic patients. The figures included in this article are useful because they are from a typical case in which results of indirect CTV were “normal” and results of direct CTV were absolutely not. It has been our experience that indirect CTV will miss considerable intraluminal details compared with direct CTV. It is important to note that good-quality magnetic resonance venography (MRV) also provides excellent images and does not use radiation or contrast, and we are slowly becoming more confident in using this imaging modality.
INDICATIONS FOR CTV
Our team uses CTV in the following cases:
• Acute iliofemoral deep vein thrombosis (DVT). We perform CTPA in a standard manner followed by indirect CTV (Figure 1). The CTPA portion enables us to rule out significant pulmonary embolus (PE) and/or right ventricular dilatation, while the CTV element shows the IVC, iliac veins, and whether the internal iliac veins or profunda femoris are thrombosed. Depending on the findings, we may use a filter or consider catheter-directed thrombolysis as opposed to standard, single-session pharmacomechanical thrombectomy.
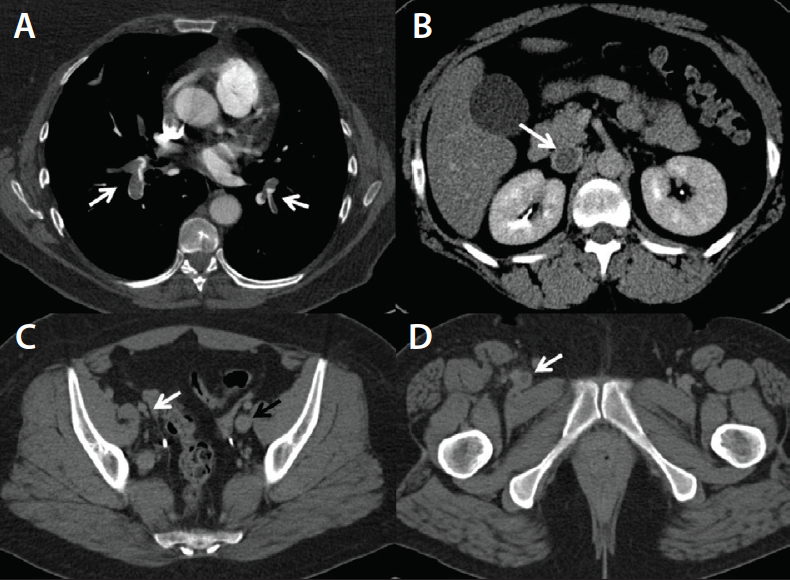
Figure 1. CTPA showing massive bilateral PE (A). Indirect CTV demonstrates acute IVC thrombosis (B), right external iliac vein scarring due to previous DVT (C), and right common femoral vein thrombosis (D).
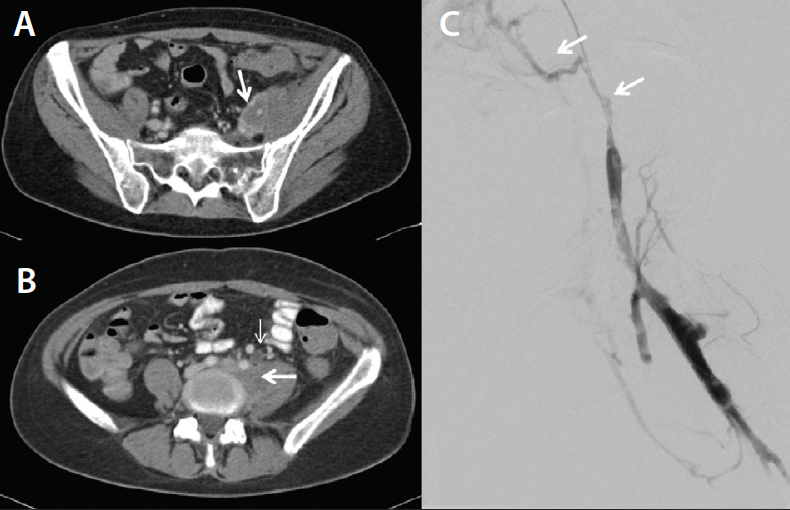
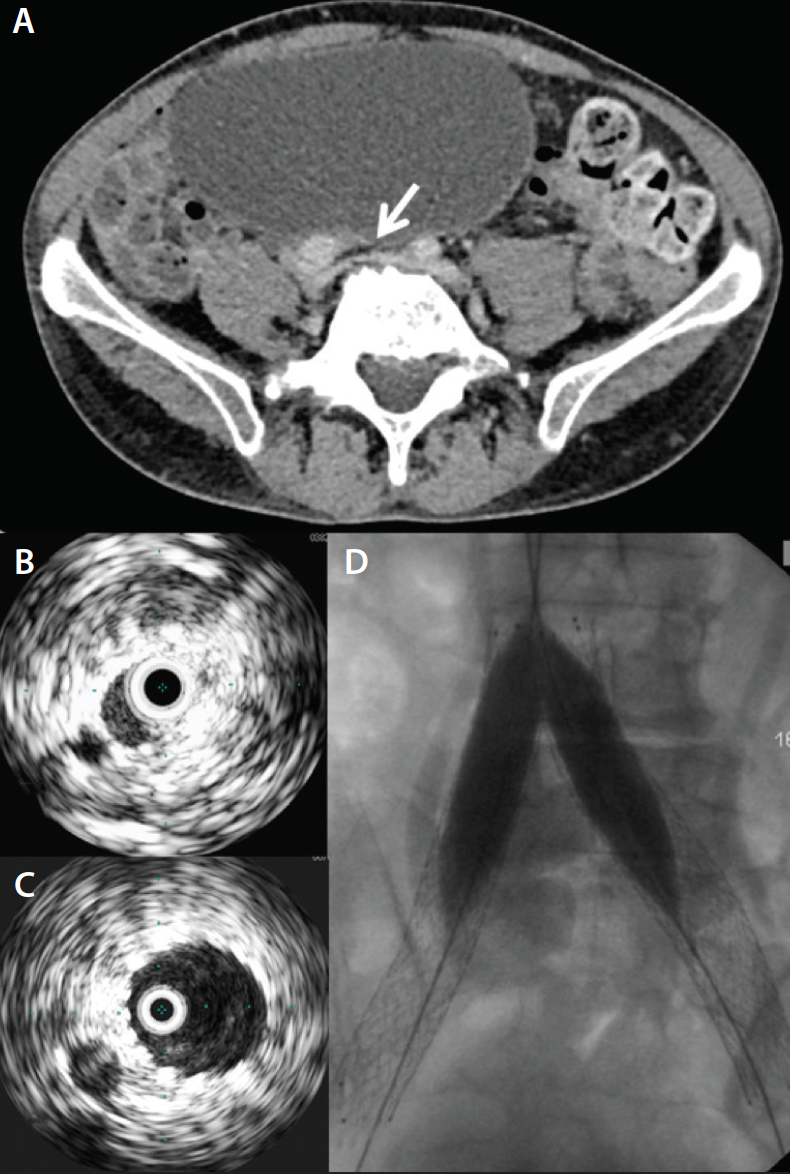
Figure 2. Indirect CTV in a patient with metastatic cervical carcinoma and left leg swelling demonstrates a pelvic mass (arrows) compressing the left common and external iliac vein (A, B). The left ureter is dilated (thin arrow) (B). Venography confirms severe stenosis of the iliac veins prior to stent placement (C).
• Malignancy/limb swelling/lymphedema. Indirect CTV is a general catch-all scan and usually is obviously abnormal or entirely normal. For example, in malignancy, there may be a large lymph node mass clearly causing venous obstruction, or in lymphedema, the scan looks absolutely normal (Figure 2).
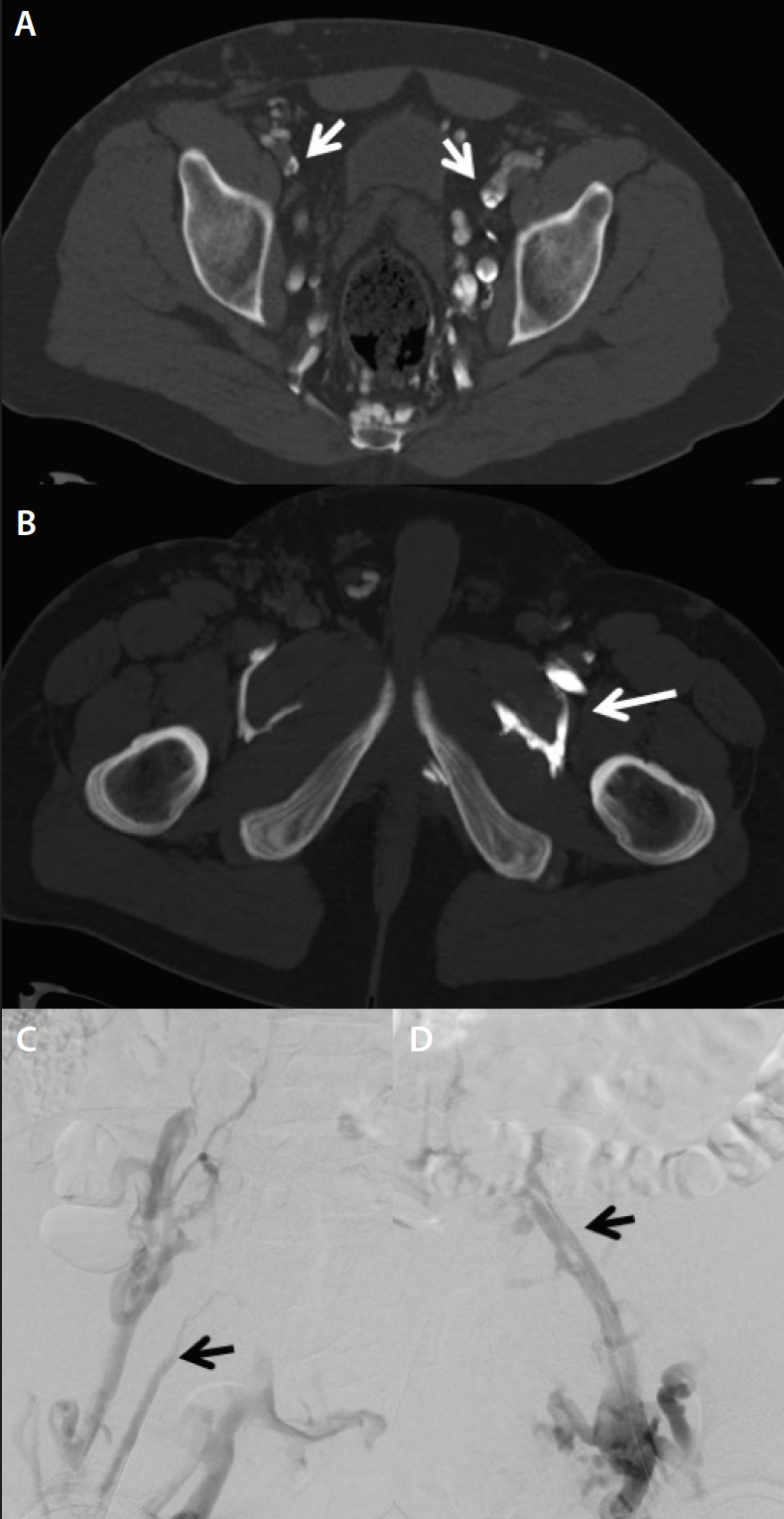
• Chronic iliofemoral venous obstruction (cases in which there is a swollen leg). Direct CTV enables detection of synechiae in postthrombotic patients; this is often missed on indirect CTV (Figure 3).
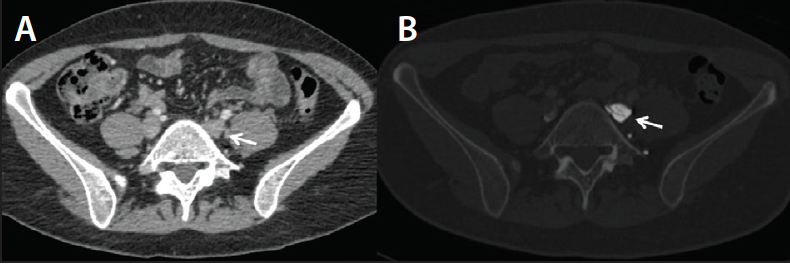
Figure 3. Unremarkable indirect CTV in a patient with left leg venous claudications and a history of DVT (A). In the same patient, direct CTV demonstrates multiple synechiae in the left common iliac vein (B).
• Bilateral swollen limbs. Indirect CTV can be used to find the causative stenosis. With adequate clinical suspicion and a somewhat abnormal CTV, intravascular ultrasound (IVUS) can be performed to confirm the stenosis (Figure 4).
• Bilateral swollen limbs and a previous history of DVT. We used bilateral direct CTV in this case (Figure 5), which technically is slightly harder to perform. Two pump injectors or a Y adaptor are needed, but this method is extremely accurate.
WHEN IS CTV NOT IDEAL?
CTV should be limited in young patients when repeated imaging is likely to be required, avoided entirely in patients who are pregnant, and when artifacts are probable (eg, patients with total hip replacements in which there is a significant metal hardening artifact).
A major disadvantage of CTV is the radiation dose, which is not trivial. Radiation exposure–induced death is a concept with which we have only recently become familiar. Unlike elderly patients with a smoking history and peripheral artery disease, many patients with venous disease are young, female, and fertile, and radiation dose should be carefully considered.
SUMMARY
Although we frequently use CTV to diagnose venous disease, over the longer term, we are considering a switch to MRV, which is much more user dependent. It is worth talking to a center that performs “perfect” MRV (we have learned a great deal from our colleagues at Maastricht University Medical Center in Maastricht, the Netherlands).
Some operators use ultrasound almost exclusively as the initial diagnostic test, and then perform IVUS as the definitive test. IVUS is the gold standard, as the recent VIDIO trial has confirmed, but it is (minimally) invasive and essentially predisposes the patient to treatment; it is rare for a patient to undergo IVUS without receiving a stent—and we don’t agree with this philosophy.
Recommended Reading
Abdalla G, Fawzi Matuk R, Venugopal V, et al. The diagnostic accuracy of magnetic resonance venography in the detection of deep venous thrombosis: a systematic review and meta-analysis. Clin Radiol. 2015;70:858-871.
Baekgaard N, Fanelli F, O’Sullivan GJ, et al. New Horizons in Deep Venous Disease Management. Torino, Italy: Edizioni Minerva Medica; 2017.
Brambilla M, De Mauri A, Lizio D, et al. Estimated radiation risk of cancer from medical imaging in haemodialysis patients. Nephrol Dial Transplant. 2014;29:1680-1686.
Gagne PJ, Tahara RW, Fastabend CP, et al. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J Vasc Surg Lymphat Disord. 2017;5:678-687.
Ghaye B, Dondelinger RF. CT venography in an integrated diagnostic strategy of acute pulmonary embolism and venous thrombosis. In: Marincek B, Dondelinger RF, editors. Emergency Radiology. Berlin: Springer; 2007.
Ghaye B, Szapiro D, Willems V, Dondelinger RF. Pitfalls in CT venography of lower limbs and abdominal veins. AJR Am J Roentgenol. 2002;178:1465-1471.
Kalva SP, Jagannathan JP, Hahn PF, Wicky ST. Venous thromboembolism: indirect CT venography during CT pulmonary angiography—should the pelvis be imaged? Radiology. 2008;246:605-611.
Katz DS, Loud PA, Bruce D, et al. Combined CT venography and pulmonary angiography: a comprehensive review. Radiographics. 2002;22:S3-19.
Mavili E, Ozturk M, Akcali Y, et al. Direct CT venography for evaluation of the lower extremity venous anomalies of Klippel-Trénaunay syndrome. AJR Am J Roentgenol. 2009;192:W311-316.
Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002;35:694-700.
Peterson DA, Kazerooni EA, Wakefield TW, et al. Computed tomographic venography is specific but not sensitive for diagnosis of acute lower-extremity deep venous thrombosis in patients with suspected pulmonary embolus. J Vasc Surg. 2001;34:798-804.
Reichert M, Henzler T, Krissak R, et al. Venous thromboembolism: additional diagnostic value and radiation dose of pelvic CT venography in patients with suspected pulmonary embolism. Eur J Radiol. 2011;80:50-53.
Sampson FC, Goodacre SW, Thomas SM, van Beek EJ. The accuracy of MRI in diagnosis of suspected deep vein thrombosis: systematic review and meta-analysis. Eur Radiol. 2007;17:175-181.
Ana Marija Alduk, MD, PhD
Department of Diagnostic and Interventional Radiology
University Hospital Center
Zagreb, Croatia
aalduk@gmail.com
Disclosures: Unavailable at the time of publication.
Gerard O’Sullivan, MD
Department of Interventional Radiology
University College Hospital
Galway, Ireland
gerard.osullivan2@hse.ie
Disclosures: Unavailable at the time of publication.
Advertisement
Advertisement